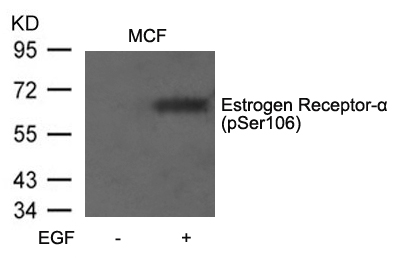
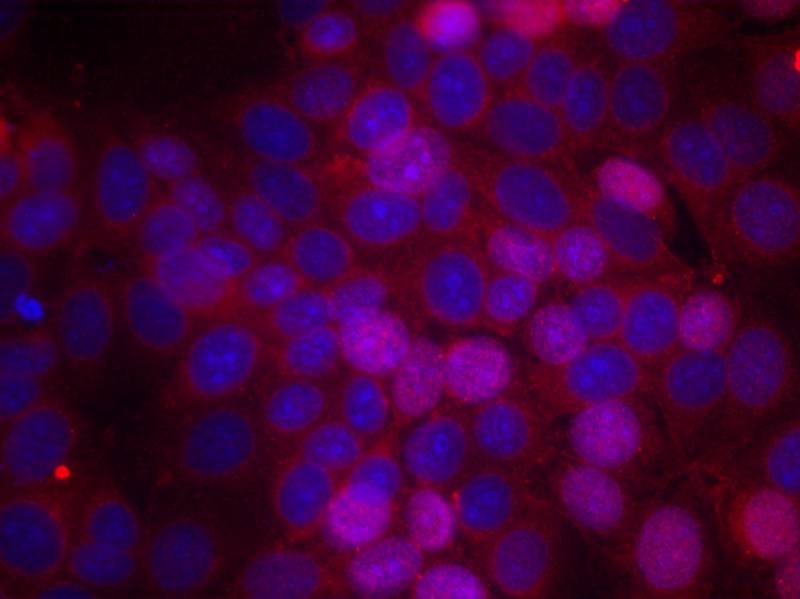
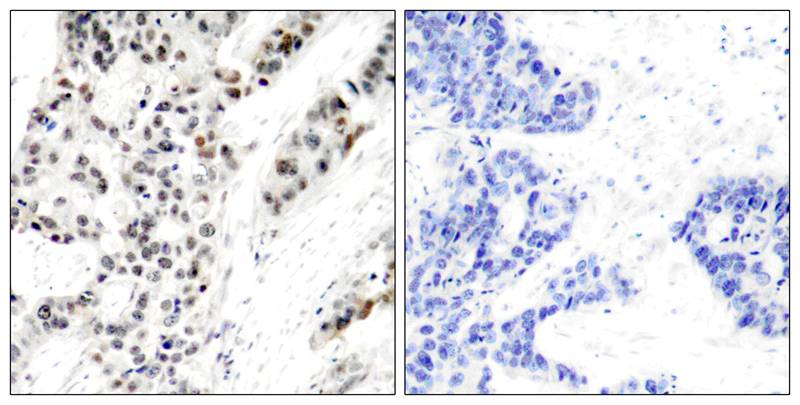
| WB | 咨询技术 | Human,Mouse,Rat |
| IF | 咨询技术 | Human,Mouse,Rat |
| IHC | 1/50-1/100 | Human,Mouse,Rat |
| ICC | 1/100-1/200 | Human,Mouse,Rat |
| FCM | 咨询技术 | Human,Mouse,Rat |
| Elisa | 咨询技术 | Human,Mouse,Rat |
| Aliases | ER; ESR; ESR1; ESTR; ESTRA |
| Entrez GeneID | 2099; |
| WB Predicted band size | 66kDa |
| Host/Isotype | Rabbit IgG |
| Antibody Type | Primary antibody |
| Storage | Store at 4°C short term. Aliquot and store at -20°C long term. Avoid freeze/thaw cycles. |
| Species Reactivity | Human,Mouse,Rat |
| Immunogen | Peptide sequence around phosphorylation site of serine106 (S-P- S(p)-P-L) derived from Human Estrogen Receptor-a. |
| Formulation | Purified antibody in PBS with 0.05% sodium azide. |
+ +
以下是关于Estrogen Receptor-α (Phospho-Ser106)抗体的3篇参考文献(基于公开知识,非实时数据库检索):
1. **"CDK7-dependent phosphorylation of estrogen receptor-α on Ser106 promotes tamoxifen resistance in breast cancer"**
- **作者**: Wang et al.
- **摘要**: 研究揭示CDK7介导的ERα Ser106磷酸化通过增强受体转录活性,导致乳腺癌对他莫昔芬治疗的耐药性。
2. **"Phosphorylation of estrogen receptor α at serine 106 modulates its ubiquitination and proteasomal degradation"**
- **作者**: Zheng et al.
- **摘要**: 发现Ser106磷酸化通过促进ERα与E3泛素连接酶的相互作用,增加其泛素化依赖的蛋白酶体降解,影响激素敏感性。
3. **"Kinase-specific phosphorylation of estrogen receptor-α at serine 106 regulates ligand-independent transcriptional activity"**
- **作者**: Thomas et al.
- **摘要**: 证实MAPK和AKT等激酶对ERα Ser106的磷酸化可激活非配体依赖的转录活性,促进乳腺癌细胞增殖。
4. **"Clinical significance of phosphorylated ERα (Ser106) in endocrine therapy-resistant metastatic breast cancer"**
- **作者**: Sanchez et al.
- **摘要**: 通过临床样本分析,发现Phospho-Ser106 ERα的高表达与内分泌治疗耐药性和患者预后不良显著相关。
注:以上内容基于领域内常见研究方向概括,具体文献需通过PubMed/Google Scholar以关键词检索确认。
Estrogen receptor-alpha (ERα) is a nuclear hormone receptor critical for mediating estrogen signaling in various tissues, including the breast, uterus, and cardiovascular system. Phosphorylation of ERα at specific serine residues, such as Ser106. dynamically regulates its transcriptional activity, protein stability, and interactions with co-regulators. The phosphorylation at Ser106 is associated with ligand-independent receptor activation and has been implicated in modulating ERα’s response to growth factor signaling pathways, including MAPK and CDK2. This post-translational modification may influence receptor dimerization, DNA binding, or recruitment of transcriptional machinery, thereby affecting estrogen-dependent gene expression.
Antibodies targeting ERα phosphorylated at Ser106 (Phospho-Ser106) are essential tools for studying the functional regulation of ERα in physiological and pathological contexts. They are widely used in techniques like Western blotting, immunohistochemistry, and immunofluorescence to detect site-specific phosphorylation status in cell lines, tissues, or clinical samples. Research utilizing these antibodies has highlighted the role of Ser106 phosphorylation in breast cancer progression, particularly in resistance to endocrine therapies like tamoxifen. Aberrant phosphorylation at this site may correlate with aggressive tumor behavior or poor prognosis, offering insights into mechanisms underlying therapeutic resistance.
These antibodies enable researchers to explore the crosstalk between ERα signaling and other oncogenic pathways, aiding the development of targeted strategies to overcome treatment resistance in hormone-dependent cancers.
×