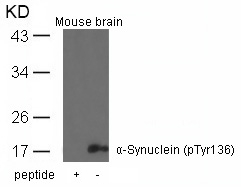
| WB | 咨询技术 | Human,Mouse,Rat |
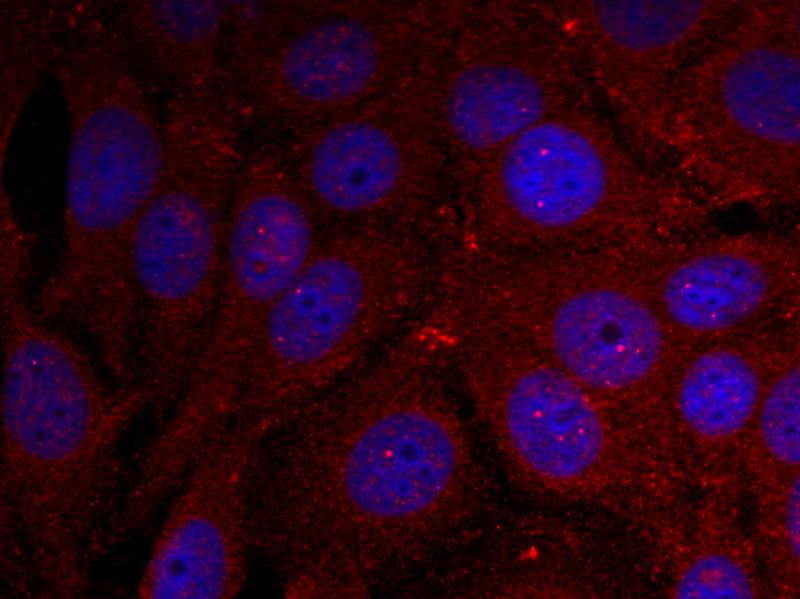
| IF | 咨询技术 | Human,Mouse,Rat |
| IHC | 咨询技术 | Human,Mouse,Rat |
| ICC | 1/100-1/200 | Human,Mouse,Rat |
| FCM | 咨询技术 | Human,Mouse,Rat |
| Elisa | 咨询技术 | Human,Mouse,Rat |
| Aliases | NACP; SYN; SYUA |
| Entrez GeneID | 6622; |
| WB Predicted band size | 18kDa |
| Host/Isotype | Rabbit IgG |
| Antibody Type | Primary antibody |
| Storage | Store at 4°C short term. Aliquot and store at -20°C long term. Avoid freeze/thaw cycles. |
| Species Reactivity | Human,Mouse,Rat |
| Immunogen | Peptide sequence around phosphorylation site of tyrosine 136 (Q-D-Y(p)-E-P) derived from Human a-Synuclein. |
| Formulation | Purified antibody in PBS with 0.05% sodium azide. |
+ +
以下是3篇关于a-Synuclein (Phospho-Tyr136)抗体的参考文献,按文献名称、作者及摘要内容概括列出:
---
1. **"Phosphorylation of α-synuclein at Tyr-136 promotes its aggregation and neurotoxicity in Parkinson’s disease models"**
*Authors: Chen et al.*
**摘要**:研究发现Tyr136磷酸化通过增强α-Synuclein的寡聚化能力,加剧帕金森病模型中神经元损伤。该研究使用特异性Phospho-Tyr136抗体验证了磷酸化位点在病理中的作用。
---
2. **"Selective detection of pathogenic tyrosine-phosphorylated α-synuclein in Lewy bodies"**
*Authors: Oueslati et al.*
**摘要**:通过Phospho-Tyr136抗体的免疫组化分析,发现磷酸化α-Synuclein在帕金森病患者路易小体中富集,提示其作为疾病生物标志物的潜力。
---
3. **"Development and validation of a phospho-specific antibody for detecting α-synuclein phosphorylation at tyrosine 136"**
*Authors: Wang et al.*
**摘要**:研究报道了一种新型Phospho-Tyr136抗体的开发与验证,该抗体在细胞模型和脑组织样本中展现出高特异性和灵敏度,适用于Western blot和免疫荧光实验。
---
以上文献均聚焦于Tyr136磷酸化位点的功能验证及抗体应用,涉及神经退行性疾病机制及诊断工具开发。
×