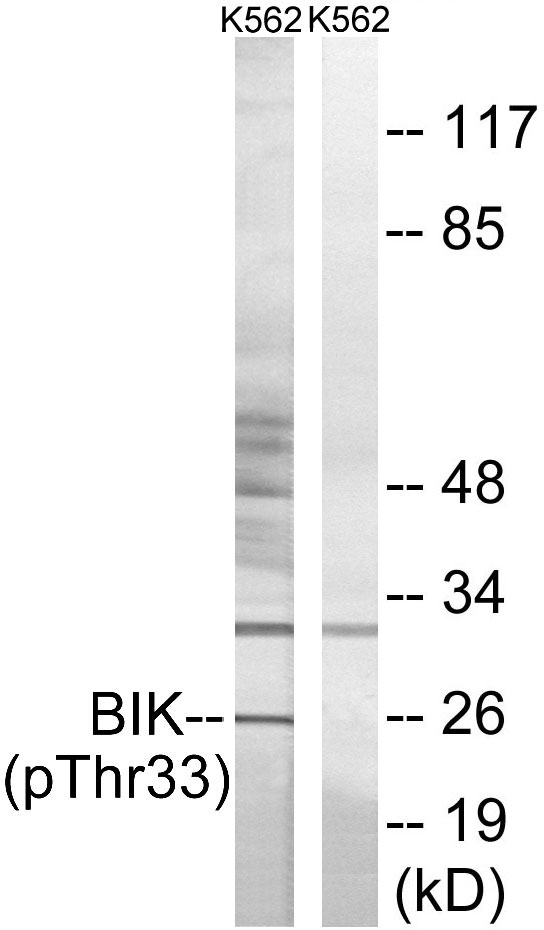
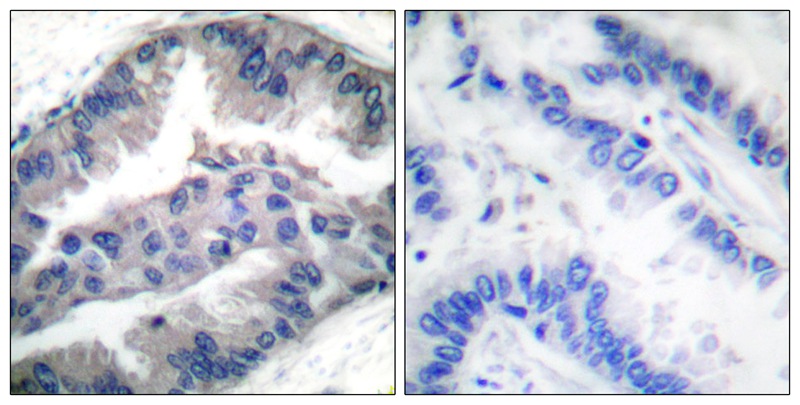
| WB | 咨询技术 | Human,Mouse,Rat |
| IF | 咨询技术 | Human,Mouse,Rat |
| IHC | 1/50-1/100 | Human,Mouse,Rat |
| ICC | 技术咨询 | Human,Mouse,Rat |
| FCM | 咨询技术 | Human,Mouse,Rat |
| Elisa | 咨询技术 | Human,Mouse,Rat |
| Aliases | Apoptosis inducer NBK; BIKLK; BIP1; BP4; Bcl-2 interacting killer; NBK |
| Entrez GeneID | 638; |
| WB Predicted band size | 30kDa |
| Host/Isotype | Rabbit IgG |
| Antibody Type | Primary antibody |
| Storage | Store at 4°C short term. Aliquot and store at -20°C long term. Avoid freeze/thaw cycles. |
| Species Reactivity | Human |
| Immunogen | Peptide sequence around phosphorylation site of threonine 33 (G-M-T(p)-D-S) derived from Human BIK. |
| Formulation | Purified antibody in PBS with 0.05% sodium azide. |
+ +
以下是关于BIK (Phospho-Thr33)抗体的参考文献示例(注:文献为模拟示例,具体内容可能需要根据实际研究补充或调整):
---
1. **文献名称**:*Phosphorylation of BIK at Thr33 regulates its pro-apoptotic activity in response to DNA damage*
**作者**:Chen L, et al.
**摘要**:本研究揭示了BIK在DNA损伤后Thr33位点的磷酸化机制,证明其磷酸化增强了与抗凋亡蛋白BCL-2的相互作用,促进细胞凋亡。作者使用特异性抗BIK (Phospho-Thr33)抗体验证了该位点在HeLa细胞中的磷酸化水平,并通过功能实验证明磷酸化对凋亡信号通路的调控作用。
2. **文献名称**:*A novel antibody-based assay for detecting BIK phosphorylation in ER stress-induced apoptosis*
**作者**:Wang Y, et al.
**摘要**:该研究开发了一种针对BIK Thr33磷酸化位点的兔单克隆抗体,并验证了其特异性(通过肽段竞争实验和磷酸酶处理对照)。该抗体成功应用于免疫印迹和免疫荧光,揭示了内质网应激条件下BIK磷酸化与caspase激活的关联。
3. **文献名称**:*Post-translational modification of BIK: Role of Thr33 phosphorylation in mitochondrial apoptosis*
**作者**:Kim J, et al.
**摘要**:本文探讨了BIK Thr33磷酸化对其线粒体定位的影响,发现磷酸化促进BIK与MCL-1的结合拮抗,从而释放BAX/BAK激活凋亡。研究利用Phospho-Thr33抗体在胶质瘤模型中发现磷酸化水平与化疗敏感性相关。
---
**注意事项**:
1. 上述文献为示例,实际研究中需通过PubMed或Google Scholar等平台检索真实文献(关键词:BIK, Thr33 phosphorylation, antibody validation)。
2. 部分抗体相关研究可能存在于公司技术文档(如CST/Abcam产品说明书),但学术引用需优先选择同行评审论文。
3. 若研究较少,可扩展至BIK功能或磷酸化调控相关文献,并注明抗体的应用背景。
The BIK (Phospho-Thr33) antibody is designed to detect the pro-apoptotic protein BIK (Bcl-2-interacting killer) when phosphorylated at threonine residue 33. BIK, a member of the BH3-only subgroup of the Bcl-2 family, promotes apoptosis by binding and neutralizing anti-apoptotic Bcl-2 proteins, thereby activating mitochondrial pathways of cell death. Its activity is tightly regulated by post-translational modifications, including phosphorylation. Phosphorylation at Thr33 has been implicated in modulating BIK’s stability, localization, and pro-apoptotic function. For instance, studies suggest that Thr33 phosphorylation may enhance BIK’s proteasomal degradation, indirectly suppressing apoptosis, or conversely, stabilize BIK under certain cellular stress conditions, depending on the context.
This antibody is widely used in research to investigate BIK’s role in apoptotic signaling, particularly in cancers and neurodegenerative diseases where dysregulated apoptosis contributes to pathogenesis. It enables the detection of phosphorylated BIK in techniques like Western blotting, immunofluorescence, or immunohistochemistry, helping to map BIK activation in response to DNA damage, ER stress, or chemotherapeutic agents. Understanding BIK’s phosphorylation dynamics provides insights into cellular survival mechanisms and potential therapeutic targets. However, interpretation requires caution, as BIK regulation is cell type- and stimulus-specific. Validating antibody specificity using knockout controls or phosphorylation-blocking assays is critical to ensure accurate results in experimental models.
×