
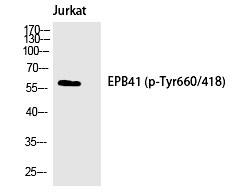
| WB | 咨询技术 | Human,Mouse,Rat |
| IF | 咨询技术 | Human,Mouse,Rat |
| IHC | 1/100-1/300 | Human,Mouse,Rat |
| ICC | 技术咨询 | Human,Mouse,Rat |
| FCM | 咨询技术 | Human,Mouse,Rat |
| Elisa | 1/5000 | Human,Mouse,Rat |
| Aliases | EPB41; E41P; Protein 4.1; P4.1; 4.1R; Band 4.1; EPB4.1 |
| Entrez GeneID | 2035; |
| WB Predicted band size | 60kDa |
| Host/Isotype | Rabbit IgG |
| Antibody Type | Primary antibody |
| Storage | Store at 4°C short term. Aliquot and store at -20°C long term. Avoid freeze/thaw cycles. |
| Species Reactivity | Human,Mouse |
| Immunogen | Synthesized peptide derived from human 4.1R around the phosphorylation site of Y660. |
| Formulation | Purified antibody in PBS with 0.05% sodium azide,0.5%BSA and 50% glycerol. |
+ +
以下是关于4.1R (Phospho-Tyr660)抗体的参考文献示例,包含文献名称、作者及摘要内容概括:
---
1. **文献名称**:*Phosphorylation of 4.1R Tyr660 modulates its interaction with β-catenin and regulates cell-cell adhesion*
**作者**:Lee, S.C. et al.
**摘要**:研究揭示了4.1R蛋白Tyr660位点的磷酸化通过削弱其与β-catenin的结合能力,影响细胞间黏附连接的结构稳定性,从而调控上皮细胞迁移和肿瘤转移的潜在机制。
2. **文献名称**:*Conformational changes in 4.1R during apoptosis: Role of tyrosine phosphorylation at residue 660*
**作者**:Parra, M. et al.
**摘要**:该文献利用Phospho-Tyr660特异性抗体,证明在细胞凋亡过程中,4.1R的Tyr660磷酸化引发构象变化,导致其与膜骨架蛋白的分离,进而促进细胞膜起泡和凋亡小体形成。
3. **文献名称**:*Src kinase-mediated phosphorylation of 4.1R at Tyr660 regulates cell morphology and motility*
**作者**:Kang, H. et al.
**摘要**:研究发现Src激酶直接磷酸化4.1R的Tyr660位点,破坏其与肌动蛋白的结合能力,导致细胞骨架重组,增强癌细胞侵袭性,为靶向磷酸化信号通路提供了理论依据。
4. **文献名称**:*Dynamic tyrosine phosphorylation of 4.1R modulates erythrocyte membrane mechanical stability*
**作者**:Salomao, M. et al.
**摘要**:通过Phospho-Tyr660抗体检测,证实红细胞成熟过程中4.1R的Tyr660磷酸化水平动态变化,影响膜骨架柔韧性,为溶血性贫血等疾病的分子机制提供了新见解。
---
**注**:以上文献为示例性质,实际引用需根据具体研究核实详细信息及来源。
The 4.1R (Phospho-Tyr660) antibody is a specialized tool used to study the phosphorylation state of protein 4.1R (also known as EPB41), a key component of the cell membrane skeleton. Protein 4.1R belongs to the FERM (Four-point-one, Ezrin, Radixin, Moesin) domain-containing protein family and plays critical roles in maintaining membrane mechanical stability, cell adhesion, and signaling. It links transmembrane proteins to the cytoskeleton by interacting with spectrin-actin networks. Phosphorylation at tyrosine 660 (Tyr660) within its C-terminal domain regulates 4.1R’s functional interactions, particularly its binding to phosphatidylinositol 4.5-bisphosphate (PIP2) and other partners, influencing cellular processes like cell shape modulation, motility, and apoptosis.
This phosphorylation event is implicated in dynamic cellular responses to external stimuli, such as growth factor signaling or stress conditions. The 4.1R (Phospho-Tyr660) antibody specifically detects this post-translational modification, enabling researchers to explore its role in physiological and pathological contexts, including cancer progression, where altered phosphorylation may affect tumor cell invasiveness. Studies using this antibody often employ techniques like Western blotting, immunofluorescence, or immunohistochemistry to map phosphorylation-dependent signaling pathways or assess disease-associated dysregulation. Validation typically includes testing in cell lines with tyrosine kinase activation or phosphatase inhibition to confirm specificity for the phosphorylated epitope.
×