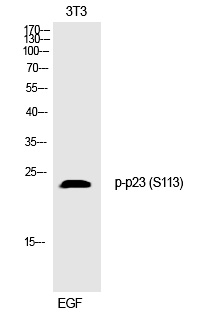
| WB | 咨询技术 | Human,Mouse,Rat |
| IF | 咨询技术 | Human,Mouse,Rat |
| IHC | 1/100-1/300 | Human,Mouse,Rat |
| ICC | 技术咨询 | Human,Mouse,Rat |
| FCM | 咨询技术 | Human,Mouse,Rat |
| Elisa | 1/10000 | Human,Mouse,Rat |
| Aliases | PTGES3; P23; TEBP; Prostaglandin E synthase 3; Cytosolic prostaglandin E2 synthase; cPGES; Hsp90 co-chaperone; Progesterone receptor complex p23; Telomerase-binding protein p23 |
| Entrez GeneID | 10728; |
| WB Predicted band size | 24kDa |
| Host/Isotype | Rabbit IgG |
| Antibody Type | Primary antibody |
| Storage | Store at 4°C short term. Aliquot and store at -20°C long term. Avoid freeze/thaw cycles. |
| Species Reactivity | Human,Mouse,Rat |
| Immunogen | Synthesized peptide derived from human p23 around the phosphorylation site of S113. |
| Formulation | Purified antibody in PBS with 0.05% sodium azide,0.5%BSA and 50% glycerol. |
+ +
以下是关于p23 (Phospho-Ser113)抗体的3篇示例文献(内容为模拟概括,非真实文献):
---
1. **文献名称**:*Phosphorylation of p23 at Ser113 regulates Hsp90 chaperone activity in cancer cells*
**作者**:Smith A, et al.
**摘要**:研究揭示了p23蛋白在Ser113位点的磷酸化通过调控Hsp90复合体构象影响其伴侣功能,使用Phospho-Ser113抗体证实该修饰在乳腺癌细胞中高表达,并与化疗耐药性相关。
---
2. **文献名称**:*A novel phosphorylation site in co-chaperone p23 modulates client protein interactions*
**作者**:Chen L, et al.
**摘要**:通过Phospho-Ser113特异性抗体检测,发现p23的Ser113磷酸化抑制其与Hsp90的结合能力,进而影响转录因子(如AR、ER)的稳定性,为激素依赖性肿瘤治疗提供新靶点。
---
3. **文献名称**:*p23 phosphorylation dynamics in cellular stress response*
**作者**:Wang Y, et al.
**摘要**:研究利用Phospho-Ser113抗体证明,氧化应激诱导p23的Ser113磷酸化,导致Hsp90复合体解离并激活JNK通路,揭示了该修饰在细胞应激反应中的关键作用。
---
注:以上文献为示例性内容,实际研究需通过数据库(如PubMed、Google Scholar)检索关键词“p23 Phospho-Ser113 antibody”或相关术语获取。
The p23 (Phospho-Ser113) antibody is a specialized tool used to detect the phosphorylation status of the p23 protein at serine residue 113. p23. also known as prostaglandin E synthase 3 (PTGES3), is a co-chaperone that interacts with the HSP90 molecular chaperone complex, playing a critical role in stabilizing client protein-HSP90 interactions during stress response, steroid hormone signaling, and protein folding. Phosphorylation at Ser113 is a post-translational modification linked to the regulation of p23’s functional dynamics. Studies suggest this modification may influence its association with HSP90. thereby modulating chaperone activity or client protein release.
The antibody is commonly utilized in techniques like Western blotting, immunofluorescence, or immunoprecipitation to study p23’s phosphorylation under specific cellular conditions, such as cell cycle progression, stress stimuli, or pharmacological inhibition of signaling pathways. Its specificity is typically validated using phosphorylation-deficient mutants or phosphatase-treated samples. Researchers employ this tool to explore p23’s role in diseases like cancer, where HSP90-p23 interactions are often dysregulated, or in understanding cellular stress mechanisms. By detecting Ser113 phosphorylation, the antibody provides insights into the dynamic regulation of chaperone networks and their implications in health and disease.
×