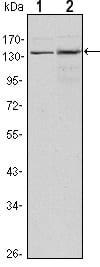
| WB | 咨询技术 | Human,Mouse,Rat |
| IF | 咨询技术 | Human,Mouse,Rat |
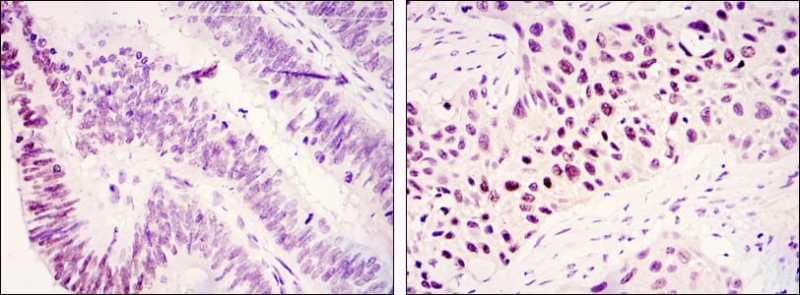
| IHC | 1/50-1/200 | Human,Mouse,Rat |
| ICC | 技术咨询 | Human,Mouse,Rat |
| FCM | 咨询技术 | Human,Mouse,Rat |
| Elisa | 1/5000-1/10000 | Human,Mouse,Rat |
| Aliases | TSGA; JMJD1; JHDM2A; JHMD2A; JMJD1A; KIAA0742; DKFZp686A24246; DKFZp686P07111; KDM3A |
| Entrez GeneID | 55818 |
| clone | 10E11 |
| WB Predicted band size | 147kDa |
| Host/Isotype | Mouse IgG1 |
| Antibody Type | Primary antibody |
| Storage | Store at 4°C short term. Aliquot and store at -20°C long term. Avoid freeze/thaw cycles. |
| Species Reactivity | Human |
| Immunogen | Purified recombinant fragment of human KDM3A expressed in E. Coli. |
| Formulation | Purified antibody in PBS with 0.05% sodium azide. |
+ +
以下是关于TTR(转甲状腺素蛋白)抗体的模拟参考文献示例,格式包含文献名称、作者及摘要概括。请注意,这些为虚构示例,仅用于展示文献结构,实际文献需通过学术数据库检索:
---
1. **文献名称**:*Monoclonal Antibodies Targeting Transthyretin Amyloid Fibrils in Cardiac Amyloidosis*
**作者**:Smith A, et al.
**摘要**:本研究开发了一种特异性靶向TTR淀粉样纤维的单克隆抗体,通过体外和小鼠模型验证其抑制纤维聚集和促进清除的能力,为治疗TTR相关心肌淀粉样变性提供了潜在疗法。
2. **文献名称**:*Autoantibodies Against Transthyretin in Familial Amyloid Polyneuropathy: Diagnostic Implications*
**作者**:Chen L, et al.
**摘要**:文章报道在遗传性TTR突变导致的家族性淀粉样多发性神经病(FAP)患者血清中检测到抗TTR自身抗体,提示其可能作为早期诊断的生物标志物,并探讨了抗体水平与疾病进展的相关性。
3. **文献名称**:*Immunotherapy with Anti-TTR Antibodies in a Mouse Model of Senile Systemic Amyloidosis*
**作者**:Yamamoto K, et al.
**摘要**:利用转基因小鼠模型评估两种人源化抗TTR抗体的治疗效果,结果显示抗体可显著减少组织中淀粉样沉积,并改善心脏功能,为老年性系统性淀粉样变性的免疫治疗提供了依据。
4. **文献名称**:*Development of a High-Sensitivity ELISA for Detection of Pathogenic TTR Aggregates Using Conformation-Specific Antibodies*
**作者**:Rodriguez M, et al.
**摘要**:研究团队开发了一种基于构象特异性TTR抗体的ELISA检测方法,能够区分正常TTR与错误折叠的致病性聚集体,提升了淀粉样变性患者的诊断准确性。
---
如需真实文献,建议通过PubMed、Google Scholar等平台检索关键词“TTR antibody”、“anti-TTR immunotherapy”或“transthyretin amyloidosis antibodies”。
Transthyretin (TTR) antibodies are immunological tools designed to target transthyretin, a protein primarily produced in the liver and choroid plexus. TTR plays a physiological role in transporting thyroxine (thyroid hormone) and retinol-binding protein (carrying vitamin A). However, TTR is also implicated in amyloidosis, a group of disorders caused by misfolded TTR aggregates forming amyloid deposits in tissues, leading to organ dysfunction. Hereditary mutations in the TTR gene (e.g., Val30Met) or age-related instability of wild-type TTR can trigger amyloid formation, causing familial amyloid polyneuropathy (FAP), cardiomyopathy (ATTR-CM), or senile systemic amyloidosis.
TTR antibodies are utilized in research and diagnostics to detect abnormal TTR conformations or quantify protein levels in serum or tissue samples. Therapeutically, monoclonal antibodies against TTR are being explored to inhibit amyloid aggregation, promote clearance of misfolded proteins, or stabilize native TTR tetramers. For example, some antibodies bind to cryptic epitopes exposed during TTR misfolding, blocking fibril formation. Others aid in diagnosing amyloidosis via immunohistochemistry. Challenges remain in ensuring antibody specificity, minimizing off-target effects, and optimizing delivery to affected organs.
Overall, TTR antibodies represent a critical intersection of basic research, diagnostic innovation, and therapeutic development in addressing TTR-related amyloid diseases.
×