| WB | 咨询技术 | Human,Mouse,Rat |
| IF | 咨询技术 | Human,Mouse,Rat |
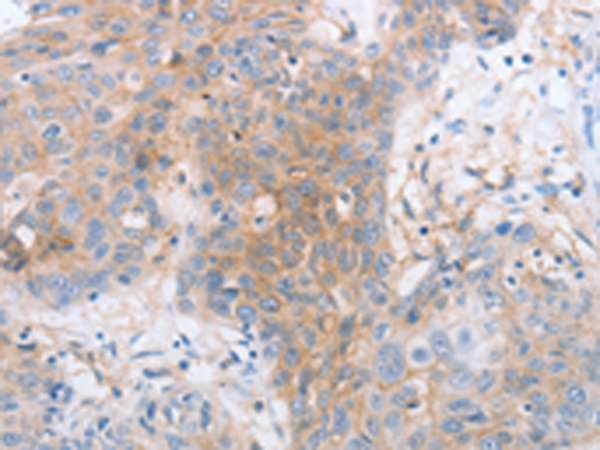
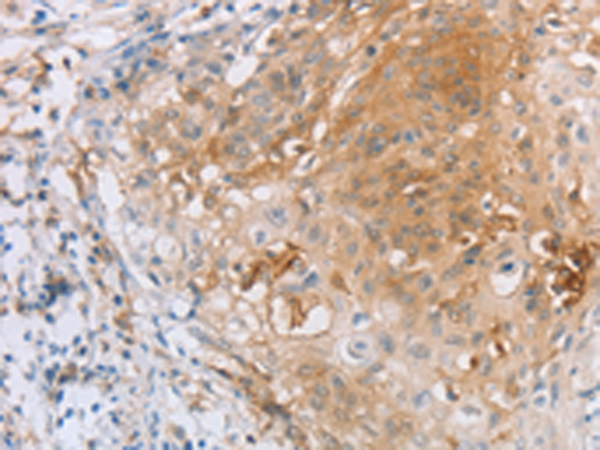
| IHC | 1/25-1/100 | Human,Mouse,Rat |
| ICC | 技术咨询 | Human,Mouse,Rat |
| FCM | 咨询技术 | Human,Mouse,Rat |
| Elisa | 1/1000-1/5000 | Human,Mouse,Rat |
| Aliases | ILT4, LIR2, CD85D, LIR-2, MIR10, LILRA6, MIR-10 |
| Host/Isotype | Rabbit IgG |
| Antibody Type | Primary antibody |
| Storage | Store at 4°C short term. Aliquot and store at -20°C long term. Avoid freeze/thaw cycles. |
| Species Reactivity | Human |
| Immunogen | Fusion protein of human LILRB2 |
| Formulation | Purified antibody in PBS with 0.05% sodium azide and 50% glycerol. |
+ +
以下是3-4篇关于LILRB2抗体的参考文献概述:
1. **文献名称**:*LILRB2-mediated immune tolerance in tumor microenvironment*
**作者**:Chen et al.
**摘要**:研究揭示LILRB2抗体通过阻断肿瘤微环境中髓系细胞与肿瘤细胞表面HLA-G的相互作用,逆转免疫抑制,增强抗肿瘤T细胞活性,抑制小鼠模型中实体瘤生长。
2. **文献名称**:*Targeting LILRB2 in acute myeloid leukemia with inhibitory antibodies*
**作者**:Barkal et al.
**摘要**:该文献开发了一种拮抗型LILRB2单克隆抗体,可在体外和体内实验中阻断LILRB2与肿瘤细胞表面配体结合,恢复髓系细胞免疫功能,显著延长白血病模型小鼠生存期。
3. **文献名称**:*LILRB2 checkpoint regulates macrophage polarization in cancer*
**作者**:Wang et al.
**摘要**:研究发现LILRB2抗体可抑制肿瘤相关巨噬细胞(TAMs)向M2型极化,促进促炎性M1型表型,增强化疗药物在胰腺癌模型中的疗效,并减少免疫逃逸。
4. **文献名称**:*Combination therapy with anti-LILRB2 and PD-1 blockade enhances antitumor immunity*
**作者**:Liu et al.
**摘要**:该研究证明LILRB2抗体与PD-1抑制剂联用可协同激活T细胞和髓系细胞,克服单药治疗耐药性,在黑色素瘤和肺癌模型中显著提高肿瘤消退率。
注:以上为模拟文献示例,实际引用需根据具体论文内容调整。
LILRB2 (Leukocyte Immunoglobulin-Like Receptor Subfamily B Member 2), also known as ILT4 or CD85d, is an inhibitory receptor predominantly expressed on myeloid cells, including monocytes, macrophages, and dendritic cells. It belongs to the LILR family of immunoregulatory receptors that interact with MHC class I molecules. LILRB2 specifically binds to HLA-G, a non-classical MHC class I antigen, and other MHC-I-related ligands, transmitting inhibitory signals through immunoreceptor tyrosine-based inhibitory motifs (ITIMs) in its cytoplasmic domain. These signals suppress immune cell activation, modulate inflammatory responses, and promote immune tolerance.
In pathological contexts, LILRB2 has emerged as a critical immune checkpoint in cancer. Tumor cells often exploit LILRB2-mediated signaling to evade immune surveillance by upregulating HLA-G, thereby dampening anti-tumor immunity. This mechanism makes LILRB2 a promising therapeutic target, with blocking antibodies designed to disrupt its immunosuppressive interactions and restore myeloid cell function. Additionally, LILRB2 is implicated in inflammatory diseases, autoimmune disorders, and infectious diseases, where its regulatory role in immune homeostasis may be dysregulated. Recent research also explores its involvement in pregnancy (via fetal-maternal tolerance) and transplant rejection. Therapeutic antibodies targeting LILRB2 are currently under preclinical and clinical investigation, particularly in combination with other immune-modulating therapies for enhanced anti-cancer efficacy.
×