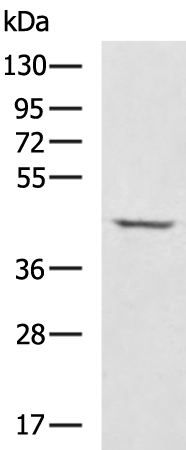
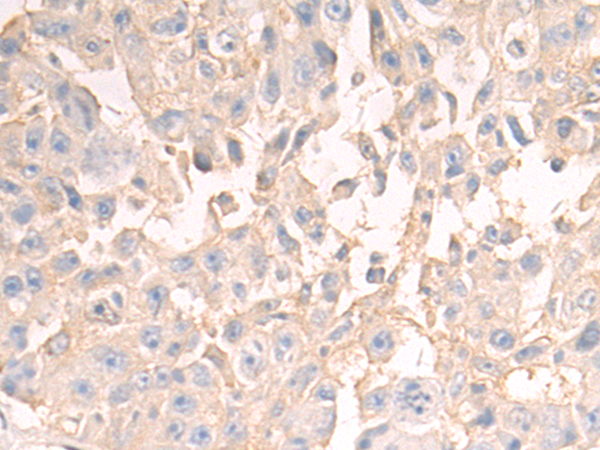
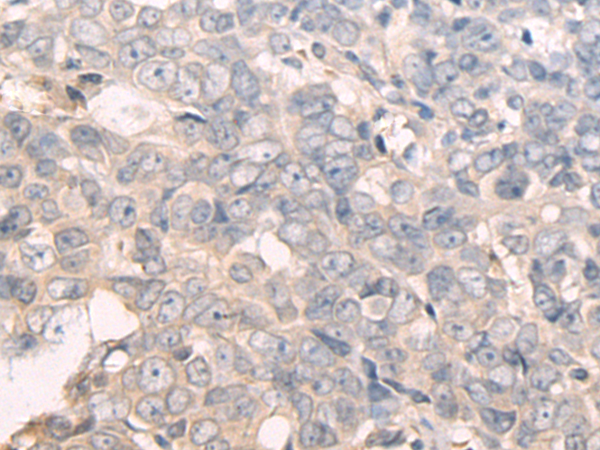
| WB | 咨询技术 | Human,Mouse,Rat |
| IF | 咨询技术 | Human,Mouse,Rat |
| IHC | 1/50-1/200 | Human,Mouse,Rat |
| ICC | 技术咨询 | Human,Mouse,Rat |
| FCM | 咨询技术 | Human,Mouse,Rat |
| Elisa | 1/5000-1/10000 | Human,Mouse,Rat |
| Aliases | ADA1 |
| WB Predicted band size | 41 kDa |
| Host/Isotype | Rabbit IgG |
| Antibody Type | Primary antibody |
| Storage | Store at 4°C short term. Aliquot and store at -20°C long term. Avoid freeze/thaw cycles. |
| Species Reactivity | Human, Rat |
| Immunogen | Fusion protein of human ADA |
| Formulation | Purified antibody in PBS with 0.05% sodium azide and 50% glycerol. |
+ +
以下是关于抗药物抗体(ADA)的3篇参考文献及其摘要概括:
---
1. **文献名称**: *Immunogenicity of Therapeutic Proteins: Influence of Aggregates*
**作者**: Jiskoot, W., et al.
**摘要**: 该综述探讨了蛋白质药物聚集物对抗原性的影响,强调聚集状态可能触发免疫反应,导致ADA产生,影响药物安全性和疗效,并提出评估策略以减少免疫原性风险。
2. **文献名称**: *Clinical significance of anti-drug antibodies to infliximab in patients with inflammatory bowel disease*
**作者**: Bartelds, G.M., et al.
**摘要**: 研究类风湿性关节炎和炎症性肠病患者中抗英夫利昔单抗抗体的发生率,发现ADA的存在与药物清除率增加及临床反应减弱显著相关,提示监测ADA对优化治疗的重要性。
3. **文献名称**: *Overcoming the challenges of immunogenicity in gene therapy with adeno-associated virus vectors*
**作者**: Mingozzi, F., & High, K.A.
**摘要**: 分析基因治疗中使用腺相关病毒(AAV)载体时ADA的作用,指出针对AAV的预存抗体可能削弱治疗效果,并提出免疫调节策略以规避ADA的负面影响。
---
如需更多细分领域(如检测技术)的文献,可进一步补充说明。
Anti-drug antibodies (ADAs) are immune proteins produced by the body in response to biologic therapies, such as recombinant proteins, monoclonal antibodies, or enzyme replacement therapies. These antibodies arise when the immune system recognizes therapeutic proteins as foreign antigens. ADA development can be influenced by factors like drug structure, patient genetics, dosing regimen, and route of administration.
ADAs are categorized as neutralizing or non-neutralizing. Neutralizing ADAs bind directly to the drug's active site, blocking its therapeutic function, while non-neutralizing ADAs may affect drug pharmacokinetics by accelerating clearance or forming immune complexes. Both types can compromise treatment efficacy, leading to reduced clinical response or loss of drug effect. In some cases, ADAs may trigger hypersensitivity reactions or autoimmune-like syndromes.
The detection and monitoring of ADAs are critical during drug development and clinical use. Techniques like ELISA, radioimmunoassay, or cell-based assays are employed to assess immunogenicity. Strategies to mitigate ADA formation include protein engineering to reduce immunogenic epitopes, optimizing dosing schedules, or co-administering immunosuppressants. However, ADA management remains challenging due to inter-patient variability in immune responses. Understanding ADA mechanisms is vital for improving biologic drug safety, efficacy, and personalized treatment approaches.
×