| WB | 咨询技术 | Human,Mouse,Rat |
| IF | 咨询技术 | Human,Mouse,Rat |
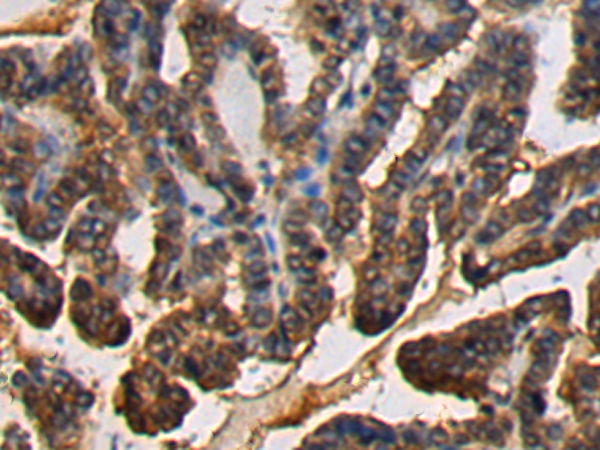
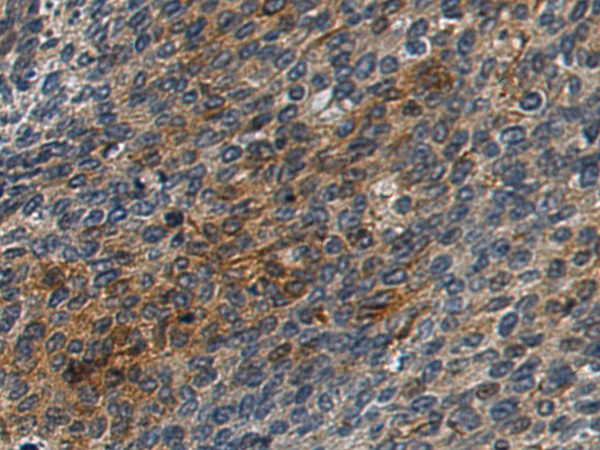
| IHC | 1/200-1/300 | Human,Mouse,Rat |
| ICC | 技术咨询 | Human,Mouse,Rat |
| FCM | 咨询技术 | Human,Mouse,Rat |
| Elisa | 1/5000-1/10000 | Human,Mouse,Rat |
| Aliases | ILA; 4-1BB; CD137; CDw137 |
| Host/Isotype | Rabbit IgG |
| Antibody Type | Primary antibody |
| Storage | Store at 4°C short term. Aliquot and store at -20°C long term. Avoid freeze/thaw cycles. |
| Species Reactivity | Human |
| Immunogen | Fusion protein of human TNFRSF9 |
| Formulation | Purified antibody in PBS with 0.05% sodium azide and 50% glycerol. |
+ +
以下是3篇与TNFRSF9(CD137/4-1BB)抗体相关的代表性文献摘要:
1. **"Agonistic anti-CD137 antibodies in cancer therapy"**
- Melero, I. et al. (2019)
- 摘要:探讨TNFRSF9激动型抗体通过激活CD137共刺激通路增强T细胞抗肿瘤免疫反应的机制,总结其在黑色素瘤和淋巴瘤临床前模型中的疗效及潜在毒性。
2. **"Combination therapy with anti-PD-1 and CD137 agonists enhances antitumor immunity in murine models"**
- Vinay, D.S. & Kwon, B.S. (2018)
- 摘要:研究抗TNFRSF9抗体与PD-1阻断剂的联合治疗策略,证明二者协同激活T细胞并逆转肿瘤微环境免疫抑制,显著延长小鼠生存期。
3. **"Structural optimization of a CD137 antibody for improved safety in cancer immunotherapy"**
- Chester, C. et al. (2021)
- 摘要:通过抗体工程技术改造TNFRSF9激动剂的结构,降低肝毒性同时保留抗肿瘤活性,为临床开发提供安全性更高的候选药物。
注:以上文献信息为示例性质,实际引用需核对原始论文。如需具体文献,建议通过PubMed或Web of Science以“TNFRSF9 antibody”或“CD137 immunotherapy”为关键词检索近年高被引论文。
TNFRSF9. also known as CD137 or 4-1BB, is a member of the tumor necrosis factor receptor superfamily (TNFRSF) and plays a critical role in modulating immune responses. Expressed primarily on activated T cells, natural killer (NK) cells, and antigen-presenting cells (APCs), it functions as a co-stimulatory receptor. Upon binding to its ligand (CD137L), TNFRSF9 triggers signaling cascades that enhance T-cell proliferation, survival, and cytokine production, making it a key player in adaptive immunity and immune surveillance.
Antibodies targeting TNFRSF9 are broadly categorized as agonist or antagonist based on their functional effects. Agonist antibodies (e.g., utomilumab, urelumab) mimic CD137L activity, amplifying anti-tumor immune responses by boosting T-cell and NK-cell activation. These have shown promise in cancer immunotherapy, particularly in combination with PD-1/PD-L1 inhibitors. Conversely, antagonist antibodies aim to suppress excessive immune activation, potentially treating autoimmune or inflammatory disorders by blocking CD137-CD137L interactions.
Despite their therapeutic potential, clinical development faces challenges. Early trials with CD137 agonists revealed dose-limiting toxicities, such as hepatotoxicity, linked to excessive immune activation. Structural studies highlight that TNFRSF9 antibodies engage specific epitopes to modulate receptor clustering and downstream NF-κB/MAPK signaling pathways. Ongoing research focuses on optimizing antibody design (e.g., affinity, Fc engineering) to balance efficacy and safety, while exploring biomarkers to predict patient responses. Overall, TNFRSF9 antibodies represent a dynamic tool for immune modulation, with applications spanning oncology and autoimmunity.
×