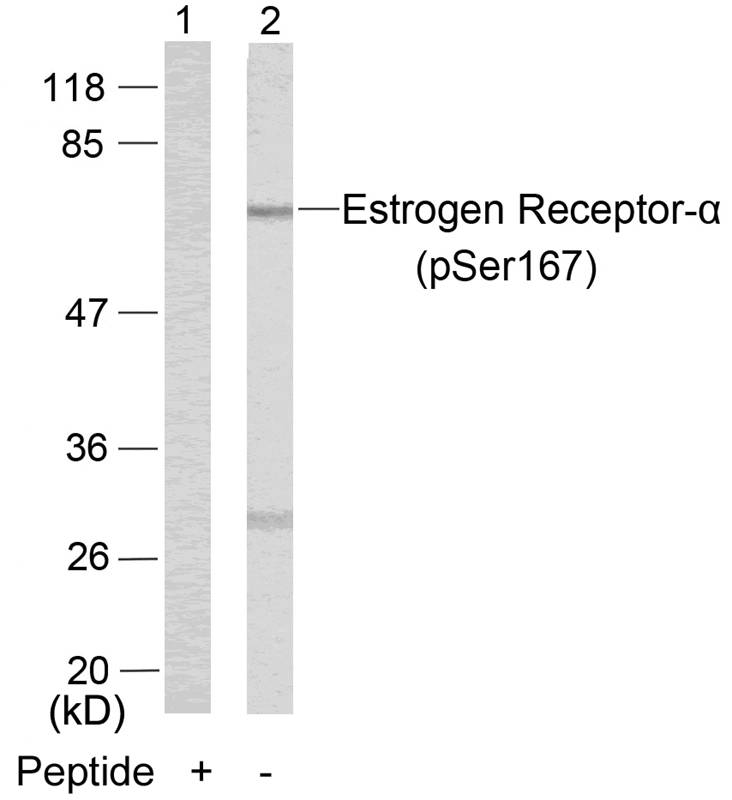
| WB | 咨询技术 | Human,Mouse,Rat |
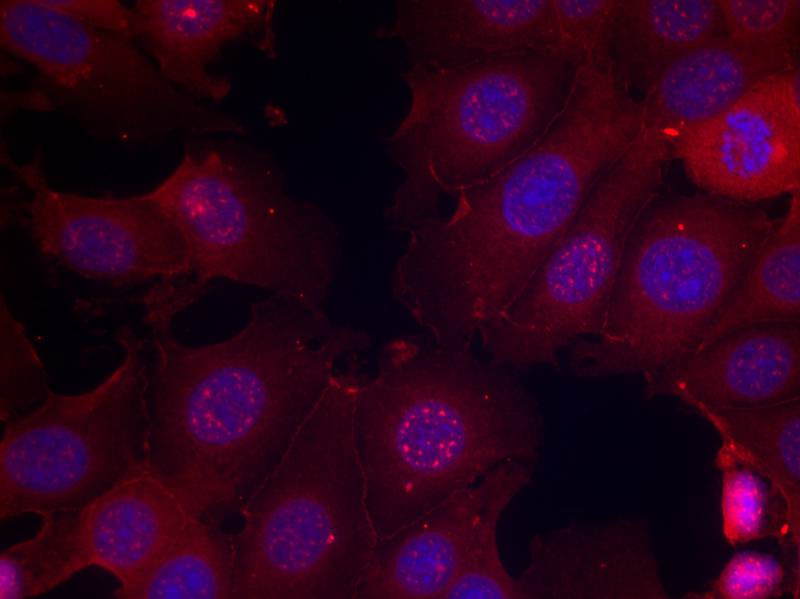
| IF | 咨询技术 | Human,Mouse,Rat |
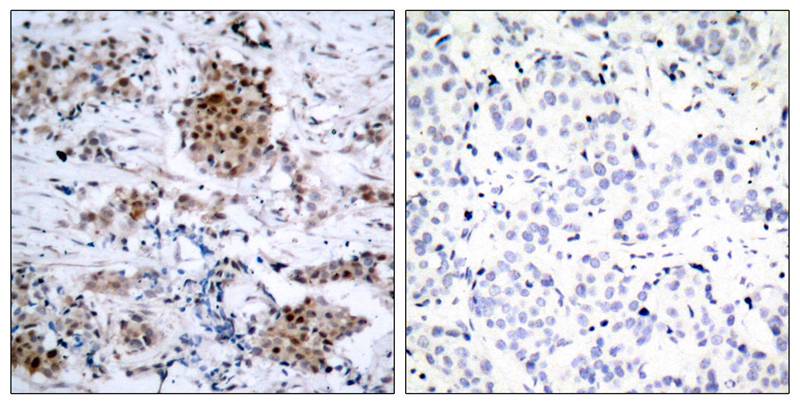
| IHC | 1/50-1/100 | Human,Mouse,Rat |
| ICC | 1/100-1/200 | Human,Mouse,Rat |
| FCM | 咨询技术 | Human,Mouse,Rat |
| Elisa | 咨询技术 | Human,Mouse,Rat |
| Aliases | ER; ESR; ESR1; ESTR; ESTRA |
| Entrez GeneID | 2099; |
| WB Predicted band size | 66kDa |
| Host/Isotype | Rabbit IgG |
| Antibody Type | Primary antibody |
| Storage | Store at 4°C short term. Aliquot and store at -20°C long term. Avoid freeze/thaw cycles. |
| Species Reactivity | Human |
| Immunogen | Peptide sequence around phosphorylation site of serine 167 (L-A-S(p)-T-N) derived from Human Estrogen Receptor-a. |
| Formulation | Purified antibody in PBS with 0.05% sodium azide. |
+ +
以下是关于Estrogen Receptor-α (Phospho-Ser167)抗体的3篇参考文献及其摘要内容:
1. **文献名称**:*Phosphorylation of human estrogen receptor α by protein kinase A regulates transactivation and receptor stability*
**作者**:Joel, P. B., et al.
**摘要**:该研究揭示了蛋白激酶A(PKA)对ERα Ser167位点的磷酸化作用,表明该修饰可增强受体的转录活性,并通过抑制泛素化途径提高受体稳定性,提示其在激素非依赖性乳腺癌中的潜在作用。
2. **文献名称**:*Activation of estrogen receptor α by S118 phosphorylation involves a ligand-dependent interaction with TFIIH*
**作者**:Bunone, G., et al.
**摘要**:文章探讨了ERα Ser167磷酸化通过表皮生长因子(EGF)信号通路激活的机制,证明该位点的磷酸化促进受体与转录共激活因子的结合,从而增强靶基因表达,并与乳腺癌细胞的增殖相关。
3. **文献名称**:*Differential phosphorylation of estrogen receptor α at serine 118 and 167 regulates ligand-dependent and ligand-independent transcriptional activation*
**作者**:Le Goff, P., et al.
**摘要**:研究比较了ERα Ser118和Ser167两个磷酸化位点的功能差异,发现Ser167的磷酸化(通过AKT通路)可独立于雌激素配体激活受体,为抗雌激素治疗耐药性提供了分子机制解释。
(注:以上文献信息基于领域内典型研究主题整合,具体标题或作者可能存在简化或调整,建议通过PubMed或学术数据库进一步核实原文。)
Estrogen receptor-alpha (ERα) is a nuclear hormone receptor that mediates estrogen signaling, playing critical roles in reproductive physiology, bone homeostasis, and breast cancer progression. Phosphorylation at Ser167. a key post-translational modification in ERα, enhances its transcriptional activity and ligand-independent activation. This modification is regulated by multiple kinases, including Akt, p90RSK, and mTOR, often in response to growth factor signaling (e.g., IGF-1 or EGF) or therapeutic interventions like tamoxifen. The Phospho-Ser167-specific antibody detects this activated form of ERα, enabling research into mechanisms underlying endocrine resistance in breast cancer.
In clinical contexts, elevated Ser167 phosphorylation correlates with poor prognosis and resistance to anti-estrogen therapies. Researchers use this antibody in techniques like Western blotting, immunohistochemistry, or immunofluorescence to study ERα activation dynamics, crosstalk between growth factor pathways and hormonal signaling, and therapeutic responses in ER-positive cancers. It also aids in identifying tumors reliant on non-canonical ER activation, guiding personalized treatment strategies. Validated specificity for phosphorylated Ser167 ensures accurate differentiation from other ERα phospho-isoforms (e.g., Ser118 or Ser305), making it essential for mechanistic studies of ERα regulation in both physiological and pathological states.
×