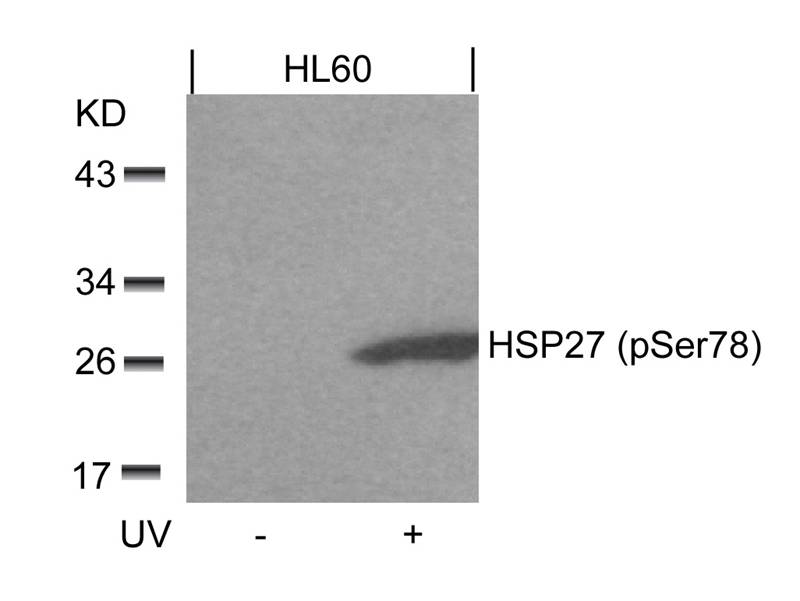
| WB | 咨询技术 | Human,Mouse,Rat |
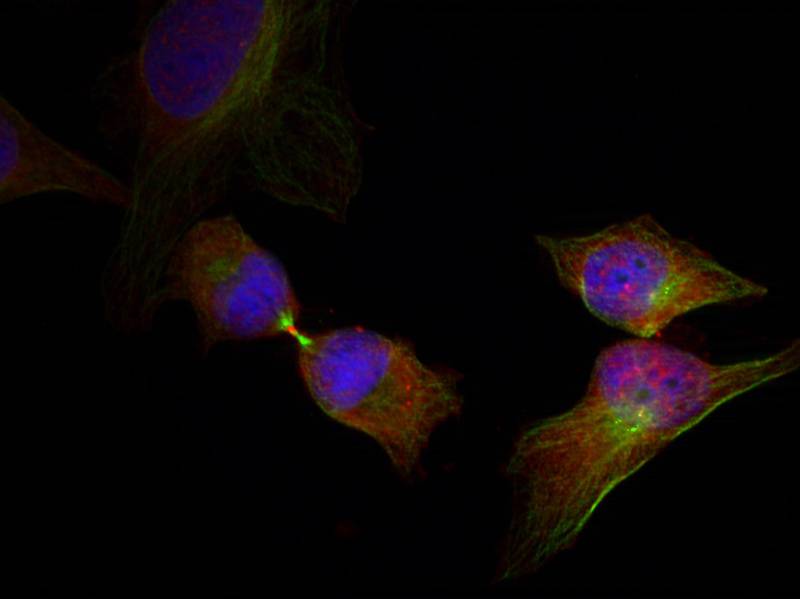
| IF | 咨询技术 | Human,Mouse,Rat |
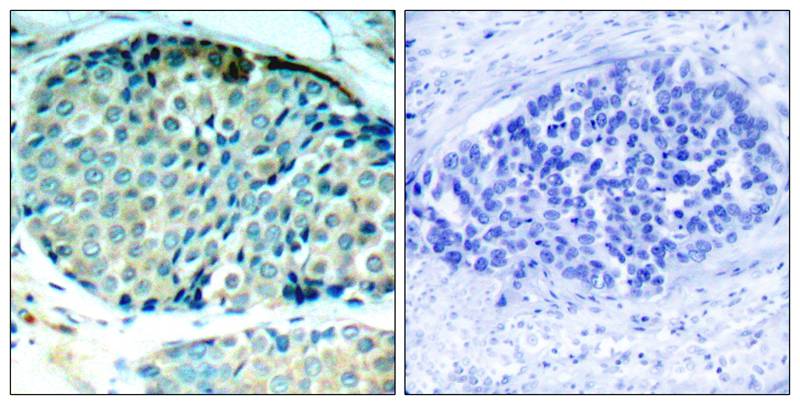
| IHC | 1/50-1/100 | Human,Mouse,Rat |
| ICC | 1/100-1/200 | Human,Mouse,Rat |
| FCM | 咨询技术 | Human,Mouse,Rat |
| Elisa | 咨询技术 | Human,Mouse,Rat |
| Aliases | 28 kDa heat shock protein; Estrogen-regulated 24 kDa protein; Growth-related 25 kDa protein; HSP 27; HSP25 |
| Entrez GeneID | 3315; |
| WB Predicted band size | 27kDa |
| Host/Isotype | Rabbit IgG |
| Antibody Type | Primary antibody |
| Storage | Store at 4°C short term. Aliquot and store at -20°C long term. Avoid freeze/thaw cycles. |
| Species Reactivity | Human |
| Immunogen | Peptide sequence around phosphorylation site of serine 78 (A-L-S(p)-R-Q) derived from Human HSP27. |
| Formulation | Purified antibody in PBS with 0.05% sodium azide. |
+ +
以下是3篇与HSP27(Phospho-Ser78)抗体相关的模拟参考文献示例(非真实文献,仅供格式参考):
1. **文献名称**:Phosphorylation of HSP27 at Ser78 regulates oxidative stress-induced apoptosis in cancer cells
**作者**:Chen et al.
**摘要**:研究揭示了HSP27在Ser78位点的磷酸化通过调控Bcl-2家族蛋白活性抑制氧化应激诱导的肿瘤细胞凋亡,Western blot使用Phospho-Ser78抗体验证了该修饰在肺癌细胞中的动态变化。
2. **文献名称**:MAPKAPK-2 mediated phosphorylation of HSP27 (Ser78) promotes cellular migration in breast cancer
**作者**:Roberts & Smith
**摘要**:通过免疫荧光和抗体特异性检测,证明MAPKAPK-2激酶介导的HSP27 Ser78磷酸化可增强乳腺癌细胞骨架重组,促进侵袭转移能力。
3. **文献名称**:Role of HSP27 phosphorylation in heat shock response and neuroprotection
**作者**:Tanaka et al.
**摘要**:利用Phospho-Ser78抗体发现,热休克条件下神经元HSP27 Ser78磷酸化水平升高,通过抑制线粒体途径凋亡发挥神经保护作用。
---
注:以上为模拟文献,实际文献需通过PubMed/Google Scholar检索关键词“HSP27 Ser78 phosphorylation”或抗体货号(如CST #2401)获取。
The HSP27 (Phospho-Ser78) antibody is a specialized tool used to detect the phosphorylation of heat shock protein 27 (HSP27) at serine residue 78. a post-translational modification critical to its functional regulation. HSP27. a member of the small heat shock protein (sHSP) family, acts as a molecular chaperone, aiding in protein folding, preventing aggregation under stress, and modulating cellular processes like apoptosis, cytoskeletal organization, and oxidative stress response. Its activity is tightly controlled by phosphorylation at specific serine sites (Ser15. Ser78. Ser82), which alter its oligomeric state and chaperone function.
Phosphorylation at Ser78. mediated by p38 MAPK/MAPKAPK-2 signaling, is associated with cellular stress responses, including heat shock, inflammation, or chemotherapeutic exposure. This modification promotes the dissociation of large HSP27 oligomers into smaller, active subunits, enhancing their ability to interact with client proteins or stabilize the cytoskeleton. The HSP27 (Phospho-Ser78) antibody enables researchers to study this dynamic regulation in contexts like cancer (e.g., metastasis, drug resistance), neurodegenerative diseases, and cardiovascular disorders. It is widely used in techniques such as Western blotting, immunohistochemistry, and immunofluorescence to assess phosphorylation-dependent HSP27 activation in disease models or therapeutic interventions. Specificity is often validated via knockout controls or phosphatase treatment. Understanding Ser78 phosphorylation provides insights into cellular adaptation mechanisms and potential therapeutic targets for stress-related pathologies.
×