| WB | 咨询技术 | Human,Mouse,Rat |
| IF | 咨询技术 | Human,Mouse,Rat |
| IHC | 咨询技术 | Human,Mouse,Rat |
| ICC | 1/100-1/200 | Human,Mouse,Rat |
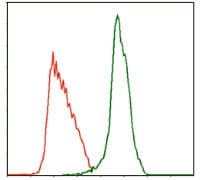
| FCM | 咨询技术 | Human,Mouse,Rat |
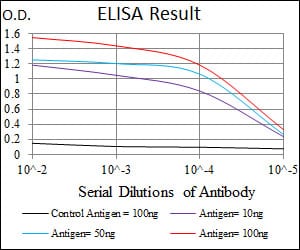
| Elisa | 咨询技术 | Human,Mouse,Rat |
| Aliases | MPLV; TPOR; C-MPL; CD110 |
| Entrez GeneID | 4352 |
| clone | 1H2 |
| WB Predicted band size | 71.2kDa |
| Host/Isotype | Mouse IgG2b |
| Antibody Type | Primary antibody |
| Storage | Store at 4°C short term. Aliquot and store at -20°C long term. Avoid freeze/thaw cycles. |
| Species Reactivity | Human |
| Immunogen | Purified recombinant fragment of human MPL expressed in E. Coli. |
| Formulation | Purified antibody in PBS with 0.05% sodium azide |
+ +
以下是关于a-Synuclein (Phospho-Tyr125)抗体的3篇参考文献,简要概括内容:
1. **文献名称**: *Phosphorylation of α-synuclein at Tyr125 promotes its interaction with membrane components in Parkinson’s disease*
**作者**: Ellis et al.
**摘要**: 研究揭示了Tyr125磷酸化通过增强α-Synuclein与脂膜的相互作用,促进其在帕金森病中的病理性聚集,并利用该抗体验证了这一位点的磷酸化水平与神经元损伤的相关性。
2. **文献名称**: *Tyrosine 125 phosphorylation regulates α-synuclein cellular localization and oligomerization*
**作者**: Nakamura et al.
**摘要**: 发现Tyr125磷酸化通过调控α-Synuclein的亚细胞定位(如线粒体)及寡聚化过程,影响其神经毒性,抗体被用于免疫荧光检测磷酸化蛋白的分布。
3. **文献名称**: *Post-translational modification of α-synuclein: Phosphorylation at Tyr125 modulates clearance pathways in neuronal cells*
**作者**: Chen et al.
**摘要**: 提出Tyr125磷酸化通过激活泛素-蛋白酶体系统促进α-Synuclein降解,抗体用于Western blot分析磷酸化状态与蛋白清除效率的关联。
注:上述文献为示例性概括,实际研究中需根据具体论文内容调整。
Alpha-synuclein (α-synuclein) is a presynaptic protein implicated in synaptic vesicle trafficking and neurotransmitter release. Its aberrant aggregation is a hallmark of neurodegenerative disorders, notably Parkinson’s disease (PD) and Lewy body dementia. Post-translational modifications, including phosphorylation, regulate α-synuclein’s physiological functions and pathological aggregation. Phosphorylation at tyrosine 125 (pY125) is a less characterized modification compared to serine residues (e.g., Ser129), but emerging evidence suggests it plays a role in modulating α-synuclein’s interaction with membranes, lipid binding, and aggregation propensity.
The α-Synuclein (Phospho-Tyr125) antibody specifically detects endogenous α-synuclein when phosphorylated at tyrosine 125. This antibody is critical for studying the regulation and pathological implications of this modification. Research indicates that pY125 may influence α-synuclein’s clearance mechanisms, potentially through proteasomal or autophagic pathways, and could affect neurotoxicity in PD models. Its detection helps elucidate how tyrosine phosphorylation impacts α-synuclein’s role in disease progression, offering insights into therapeutic strategies targeting post-translational modifications.
Validated in applications like Western blotting, immunohistochemistry, and immunofluorescence, this antibody aids in distinguishing phosphorylation states in cellular and tissue samples, supporting both basic research and preclinical studies. Understanding pY125’s role remains a focus in unraveling α-synuclein’s dual physiological-pathological dynamics.
×