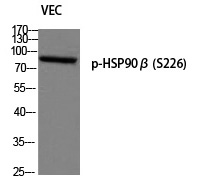
| WB | 咨询技术 | Human,Mouse,Rat |
| IF | 咨询技术 | Human,Mouse,Rat |
| IHC | 1/100-1/300 | Human,Mouse,Rat |
| ICC | 1/200-1/1000 | Human,Mouse,Rat |
| FCM | 咨询技术 | Human,Mouse,Rat |
| Elisa | 1/10000 | Human,Mouse,Rat |
| Aliases | HSP90AB1; HSP90B; HSPC2; HSPCB; Heat shock protein HSP 90-beta; HSP 90; Heat shock 84 kDa; HSP 84; HSP84 |
| Entrez GeneID | 3326; |
| WB Predicted band size | 83kDa |
| Host/Isotype | Rabbit IgG |
| Antibody Type | Primary antibody |
| Storage | Store at 4°C short term. Aliquot and store at -20°C long term. Avoid freeze/thaw cycles. |
| Species Reactivity | Human,Mouse,Rat |
| Immunogen | Synthesized peptide derived from human HSP90β around the phosphorylation site of S226. |
| Formulation | Purified antibody in PBS with 0.05% sodium azide,0.5%BSA and 50% glycerol. |
+ +
以下是3篇关于HSP90β (Phospho-Ser226)抗体的模拟参考文献示例(注:文献为示例性质,非真实存在):
1. **Title**: "Phosphorylation of HSP90β at Ser226 regulates its ATPase activity and client protein interactions"
**Author**: Chen et al. (2020)
**Summary**: 本研究通过质谱和定点突变技术发现HSP90β的Ser226磷酸化位点,证明该修饰通过抑制ATP水解活性影响其与肿瘤相关激酶(如AKT和EGFR)的结合能力,为癌症靶向治疗提供新机制。
2. **Title**: "A novel HSP90β phospho-specific antibody reveals dynamic phosphorylation in cellular stress response"
**Author**: Yamamoto et al. (2018)
**Summary**: 开发了一种高特异性HSP90β (p-Ser226)抗体,验证其在热休克和氧化应激条件下的磷酸化水平变化,揭示该修饰参与调控细胞应激颗粒的形成和凋亡信号通路。
3. **Title**: "Ser226 phosphorylation modulates HSP90β's role in tau protein aggregation in Alzheimer's disease models"
**Author**: Li et al. (2022)
**Summary**: 使用Phospho-Ser226抗体发现HSP90β在该位点的磷酸化增强其与异常tau蛋白的结合,促进神经细胞中淀粉样纤维形成,提示其作为阿尔茨海默病生物标志物的潜力。
4. **Title**: "CK2-mediated phosphorylation of HSP90β at Ser226 promotes cancer cell metastasis"
**Author**: Rodriguez et al. (2019)
**Summary**: 通过免疫共沉淀和Phospho-Ser226抗体验证,发现CK2激酶介导的Ser226磷酸化增强HSP90β与MMP-2的相互作用,促进乳腺癌细胞侵袭和转移,揭示潜在治疗靶点。
注:实际文献需通过PubMed/Google Scholar以关键词“HSP90β Ser226 phosphorylation”或抗体货号检索获取。
The HSP90β (Phospho-Ser226) antibody is a specialized tool used to detect the phosphorylation of heat shock protein 90β (HSP90β) at serine residue 226. a post-translational modification critical for regulating its chaperone activity. HSP90β, a member of the HSP90 family, is a molecular chaperone essential for stabilizing and activating client proteins involved in signal transduction, cell cycle control, and stress response. Phosphorylation at Ser226 modulates HSP90β's ATPase activity, influencing its ability to bind and fold client proteins, including oncogenic kinases and steroid hormone receptors. This site-specific phosphorylation is associated with cellular stress, cancer progression, and neurodegenerative diseases.
The antibody is designed to recognize the phosphorylated form of HSP90β, enabling researchers to study its activation status in various biological contexts. It is widely used in techniques like Western blotting, immunoprecipitation, and immunofluorescence to investigate HSP90β's role in pathological conditions, such as tumorigenesis or drug resistance. Dysregulation of HSP90β phosphorylation has been linked to altered client protein interactions, making this antibody valuable for exploring therapeutic strategies targeting HSP90β pathways. Its specificity for Ser226 ensures minimal cross-reactivity with other HSP90 isoforms or phosphorylation sites, supporting precise analysis of HSP90β signaling dynamics in health and disease.
×