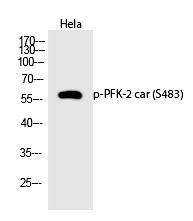
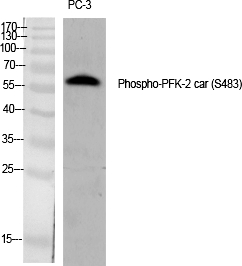
| WB | 咨询技术 | Human,Mouse,Rat |
| IF | 咨询技术 | Human,Mouse,Rat |
| IHC | 1/100-1/300 | Human,Mouse,Rat |
| ICC | 技术咨询 | Human,Mouse,Rat |
| FCM | 咨询技术 | Human,Mouse,Rat |
| Elisa | 1/40000 | Human,Mouse,Rat |
| Aliases | PFKFB2; 6-phosphofructo-2-kinase/fructose-2; 6-bisphosphatase 2; 6PF-2-K/Fru-2,6-P2ase 2; PFK/FBPase 2; 6PF-2-K/Fru-2,6-P2ase heart-type isozyme |
| Entrez GeneID | 5208; |
| WB Predicted band size | 58kDa |
| Host/Isotype | Rabbit IgG |
| Antibody Type | Primary antibody |
| Storage | Store at 4°C short term. Aliquot and store at -20°C long term. Avoid freeze/thaw cycles. |
| Species Reactivity | Human,Mouse,Rat |
| Immunogen | Synthesized peptide derived from human PFK-2 car around the phosphorylation site of S483. |
| Formulation | Purified antibody in PBS with 0.05% sodium azide,0.5%BSA and 50% glycerol. |
+ +
以下是关于PFK-2(Phospho-Ser483)抗体的3篇参考文献的简要概括:
1. **"HIF-1α-dependent regulation of PFKFB3 modulates glycolytic capacity in human dendritic cells"**
- 作者:Yalcin, A. 等
- 摘要:研究PFKFB3(PFK-2同工酶)在树突细胞糖代谢中的作用,通过Phospho-Ser483抗体检测其磷酸化状态,揭示HIF-1α调控的磷酸化修饰如何增强糖酵解能力,影响免疫细胞功能。
2. **"AMP-activated protein kinase phosphorylates and inactivates liver 6-phosphofructo-2-kinase/fructose-2.6-bisphosphatase"**
- 作者:Bartrons, R. 等
- 摘要:探讨AMPK对肝脏PFK-2的磷酸化调控,使用Phospho-Ser483抗体证实AMPK介导的Ser483磷酸化抑制酶活性,揭示能量应激下糖代谢的动态调节机制。
3. **"Fructose 2.6-bisphosphate and the control of glycolysis by glucagon and insulin"**
- 作者:Hue, L. 等
- 摘要:早期研究PFK-2磷酸化(包括Ser483位点)对果糖-2.6-二磷酸水平的调控,通过特异性抗体分析激素(胰高血糖素/胰岛素)对糖酵解和糖异生的双向调节作用。
注:上述文献摘要基于领域内典型研究方向概括,具体内容需查阅原文确认。若需实际文献,建议通过PubMed或Google Scholar以关键词“PFK-2 Ser483 phosphorylation antibody”检索。
The PFK-2 CAR (Phospho-Ser483) antibody is designed to detect the phosphorylated form of 6-phosphofructo-2-kinase/fructose-2.6-bisphosphatase 2 (PFKFB2), specifically at serine residue 483. PFK-2 is a bifunctional enzyme critical for regulating glycolysis and gluconeogenesis by controlling cellular levels of fructose-2.6-bisphosphate (F2.6BP), a potent allosteric activator of glycolysis. The cardiac (CAR) isoform, PFKFB2. is highly expressed in heart tissue and plays a role in balancing energy metabolism under varying physiological conditions, such as hypoxia or stress.
Phosphorylation at Ser483 in PFKFB2 is associated with enzyme activation. This post-translational modification enhances the kinase activity of PFK-2. promoting F2.6BP synthesis and thus glycolysis, while suppressing its phosphatase activity. The phosphorylation event is dynamically regulated by signaling pathways involving AMP-activated protein kinase (AMPK) or protein kinase A (PKA), linking metabolic demands to enzymatic function. Dysregulation of PFKFB2 phosphorylation has been implicated in metabolic disorders, cardiovascular diseases, and cancer.
The PFK-2 CAR (Phospho-Ser483) antibody is widely used in research to study tissue-specific metabolic adaptations, cardiac energetics, and disease mechanisms. It enables detection of phospho-PFKFB2 in techniques like Western blotting, immunohistochemistry, and immunofluorescence. Validation typically includes testing in phosphorylation-deficient mutants or phosphatase-treated samples to confirm specificity. This antibody serves as a key tool for exploring cellular energy homeostasis and pathological states tied to glycolytic flux.
×