关键信息
-
基因名
BAFFR/TNFRSF13C
-
简介
BAFFR/TNFRSF13C Protein, a B-cell receptor, specifically recognizes TNFSF13B/TALL1/BAFF/BLyS, playing a crucial role in promoting the survival of mature B-cells and enhancing the B-cell response, contributing to a healthy immune system. BAFFR/TNFRSF13C Protein, Human (HEK293, hFc-Flag) is the recombinant human-derived BAFFR/TNFRSF13C protein, expressed by HEK293 , with C-hFc, C-Flag labeled tag.
- 应用
-
别名
Tumor necrosis factor receptor superfamily member 13C; BAFF-R; CD268; TNFRSF13C; BR3
-
种属
Human
-
表达系统
HEK293
-
标签
C-hFc;C-Flag
-
纯度
Greater than 90% as determined by SDS-PAGE.
-
蛋白编号
Q96RJ3-1
-
表达区间
S7-A71
-
蛋白长度
Partial
-
内毒素
< 1.0 EU per μg protein as determined by the LAL method.
-
性状
Freeze-dried powder
-
缓冲液
PBS, pH7.4, containing 0.01% SKL, 1mM DTT, 5% Trehalose and Proclin300.
-
复溶方法
Reconstitute in ddH2O to a concentration of 0.1-0.5 mg/mL. Do not vortex.
- 个性化定制
-
稳定性测试
The thermal stability is described by the loss rate. The loss rate was determined by accelerated thermal degradation test, that is, incubate the protein at 37℃ for 48h, and no obvious degradation and precipitation were observed. The loss rate isless than 8% within the expiration date under appropriate storage condition.
-
保存条件 & 期限
Samples are stable for up to twelve months from date of receipt at -20℃ to -80℃. Store it under sterile conditions at -20℃ to -80℃. It is recommended that the protein be aliquoted for optimal storage. Avoid repeated freeze-thaw cycles.
-
运输条件
In general, recombinant proteins are supplied as lyophilized powder and shipped at ambient temperature. For bulk packages, the proteins are provided as frozen liquid and shipped with blue ice, unless otherwise requested by the customer.
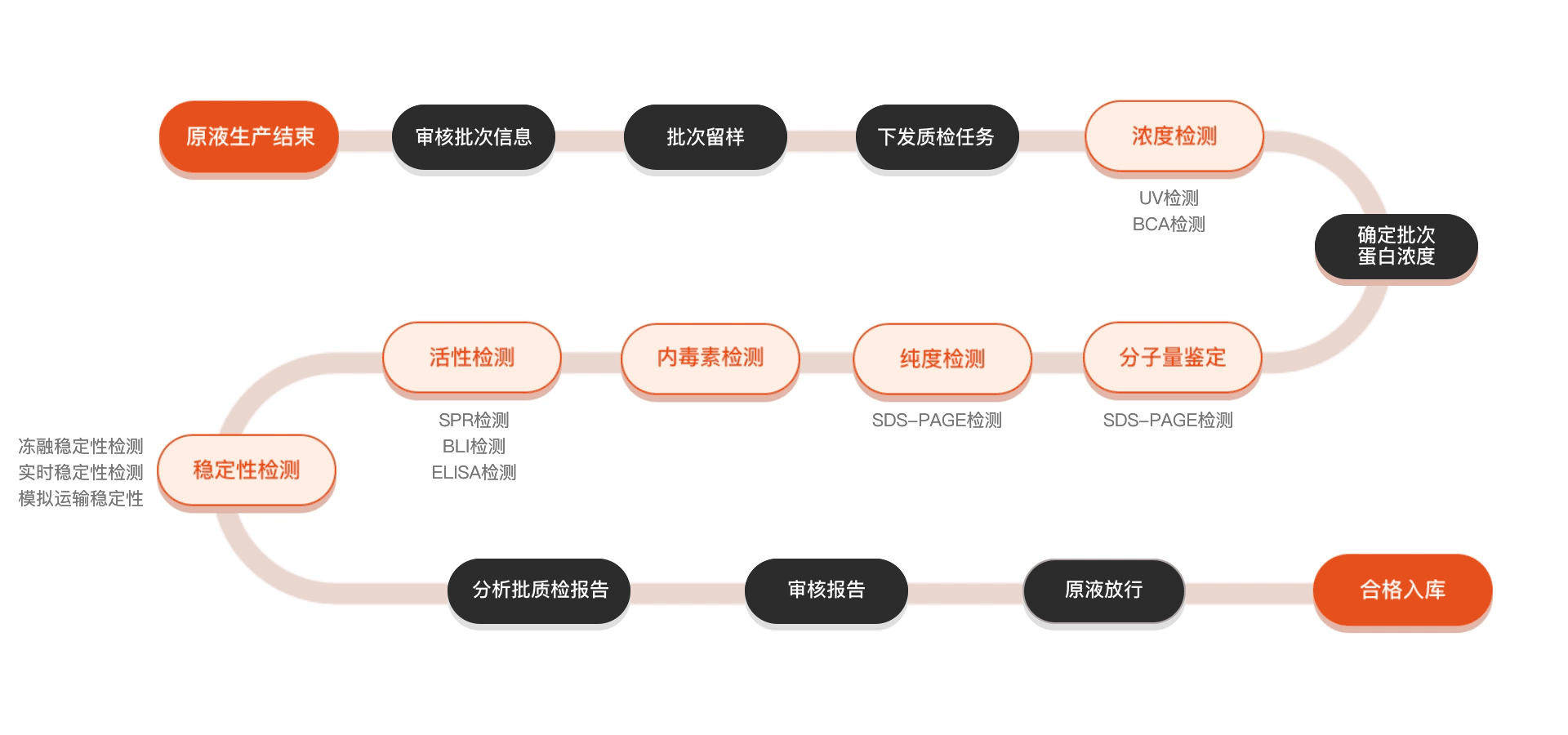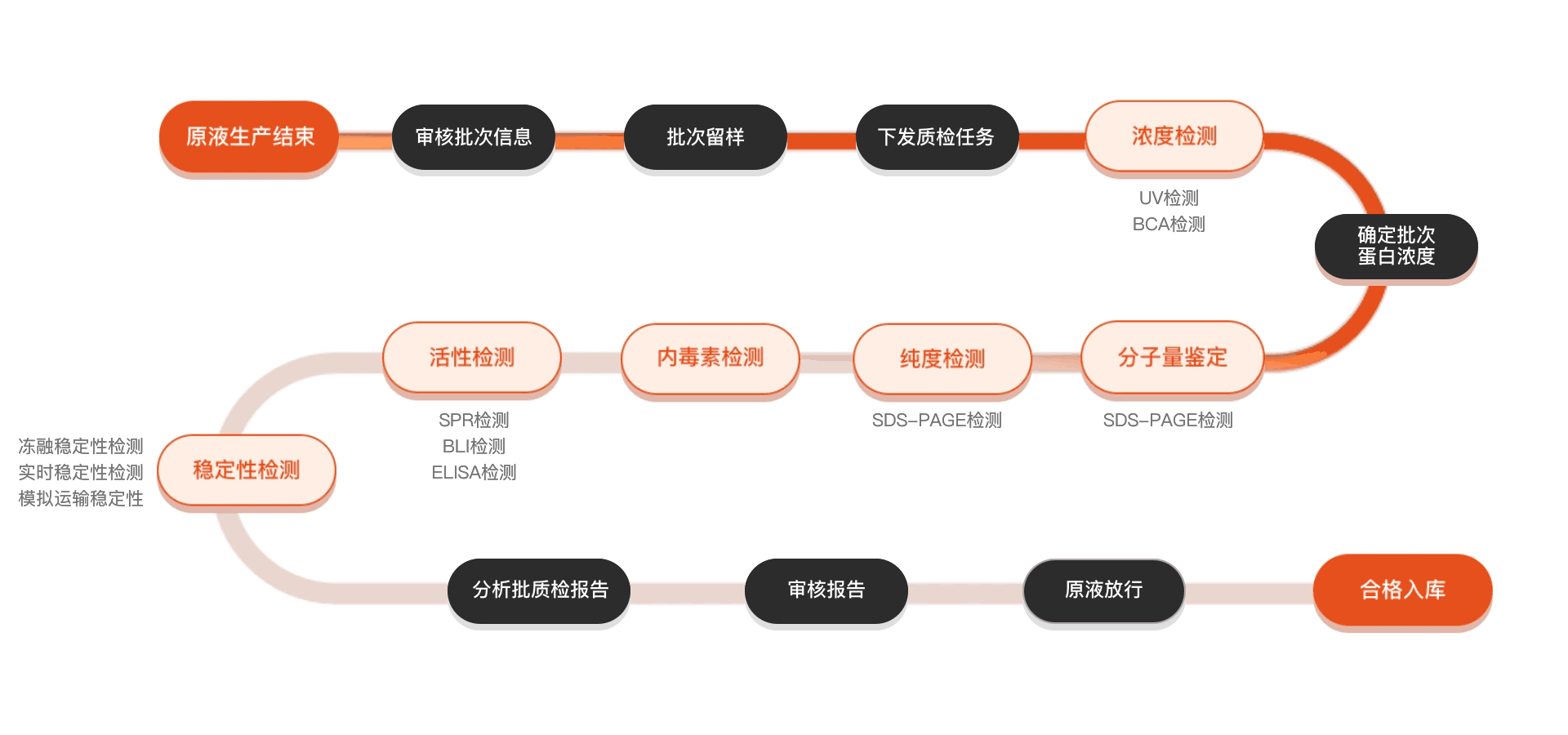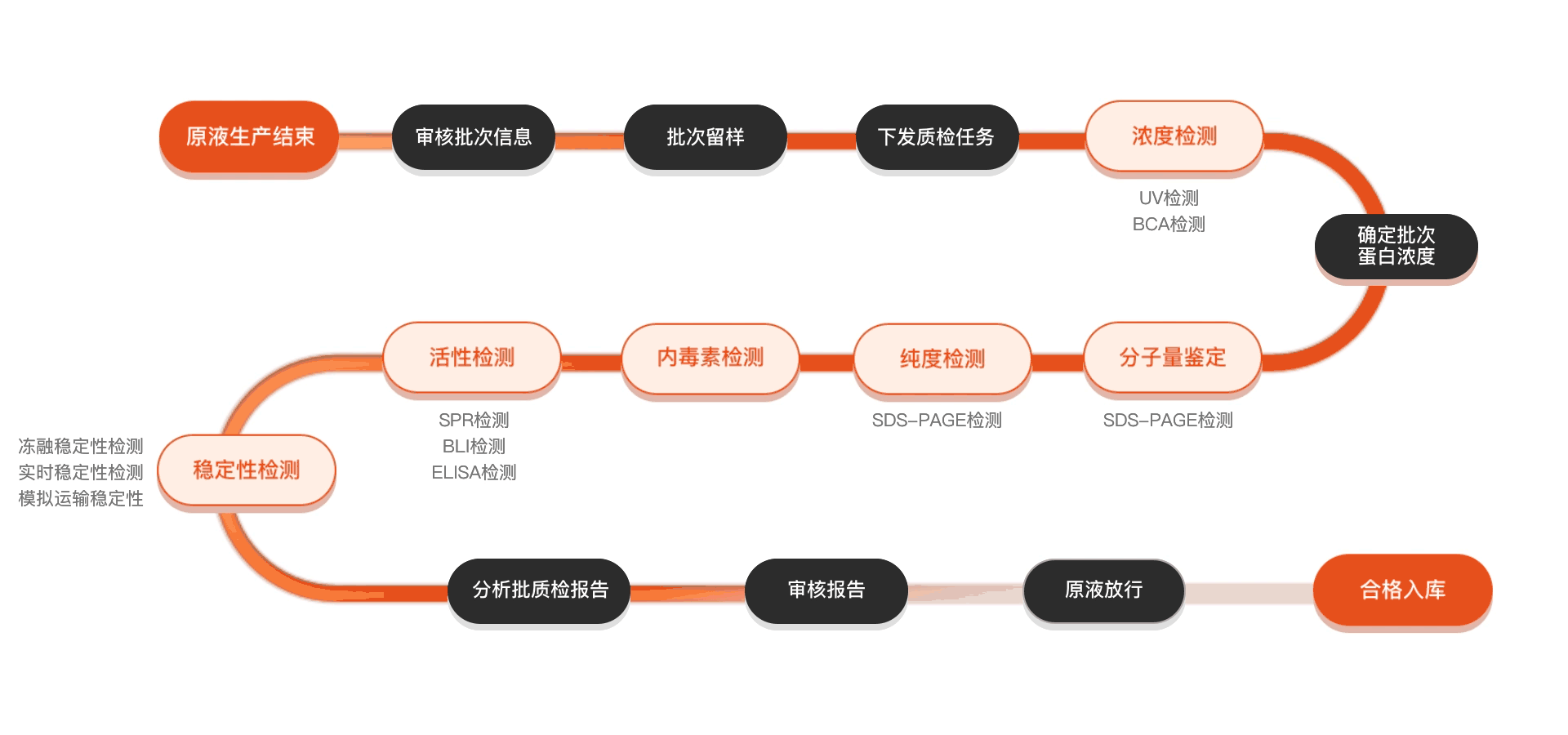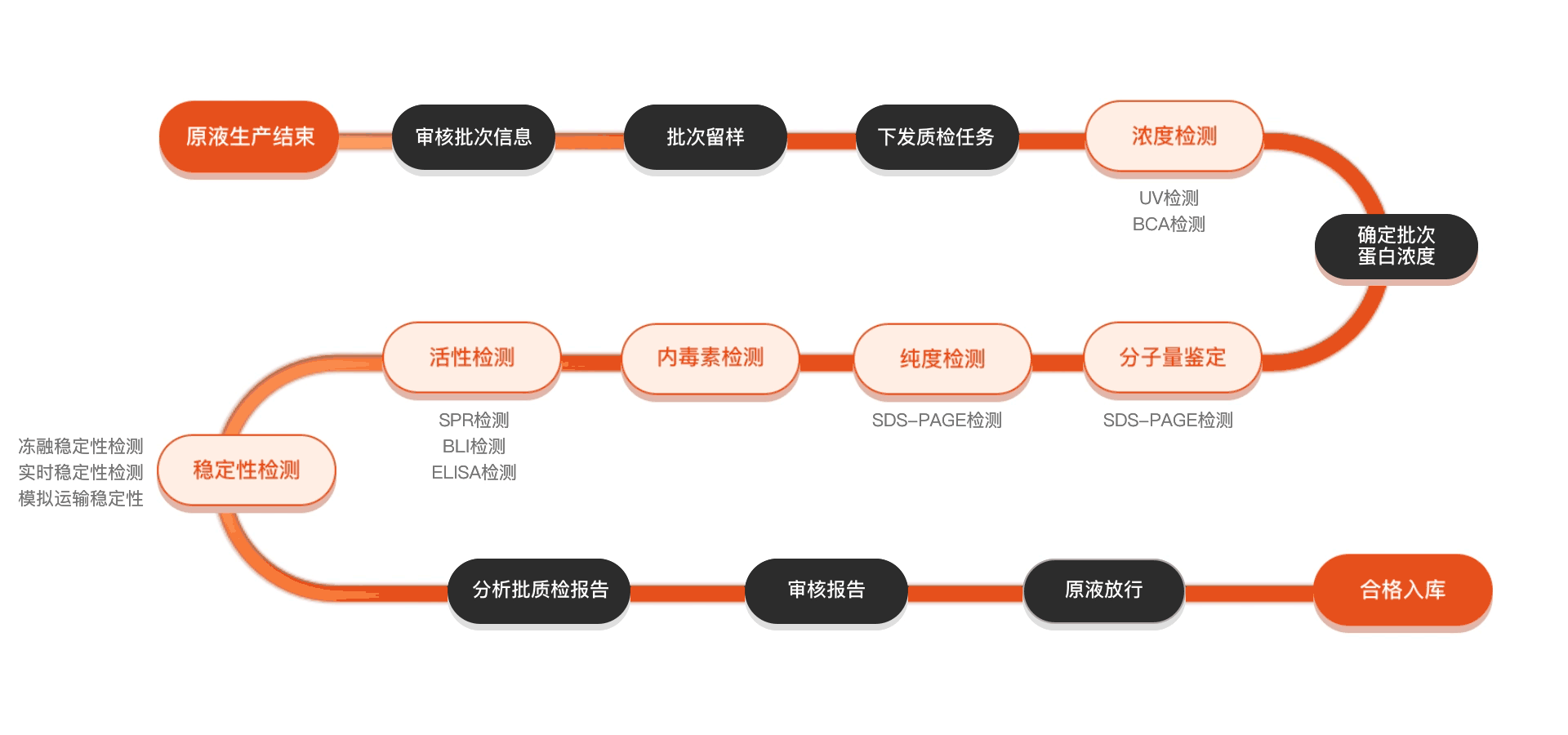
质检流程